Page 116 - The Vasculitides, Volume 1: General Considerations and Systemic Vasculitis
P. 116
92 Alan D. Salama and Mark A. Little
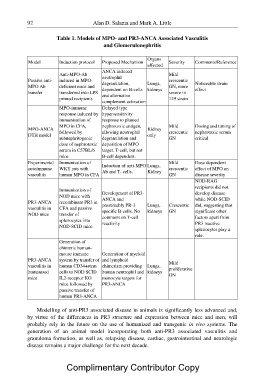
Table 1. Models of MPO- and PR3-ANCA Associated Vasculitis
and Glomerulonephritis
Model Induction protocol Proposed Mechanism Organs Severity Comments/Reference
affected
Passive anti-
MPO Ab Anti-MPO-Ab ANCA induced Mild
transfer induced in MPO- neutrophil crescentic
GN, more
MPO-ANCA deficient mice and degranulation, Lungs, severe in Noticeable strain
DTH model transferred into LPS 129 strain effect
primed recipients dependent on B-cells kidneys
Experimental
autoimmune and alternative
vasculitis
complement activation
PR3-ANCA
vasculitis in MPO-immune Delayed type
NOD mice
response induced by hypersensitivity
PR3-ANCA
vasculitis in immunisation of response to planted
humanised
mice MPO in CFA, nephrotoxic antigen, Kidney Mild Dosing and timing of
followed by allowing neutrophil only crescentic nephrotoxic serum
subnephritogenic degranulation and GN critical
dose of nephrotoxic deposition of MPO
serum in C57BL/6 target. T-cell, but not
mice B-cell dependent.
Immunisation of Induction of anti-MPO Lungs, Mild Dose dependent
WKY rats with crescentic effect of MPO on
human MPO in CFA Ab and T- cells. Kidney GN disease severity
NOD-RAG
Immunisation of Development of PR3- Crescentic recipients did not
NOD mice with ANCA and GN develop disease
recombinant PR3 in presumably PR-3 Lungs, while NOD-SCID
CFA and passive specific B-cells. No kidneys did, suggesting that
transfer of comment on T-cell significant other
splenocytes into reactivity factors apart from
NOD-SCID mice PR3 reactive
splenocytes play a
role.
Generation of
chimeric human-
mouse immune Generation of myeloid
system by transfer of and lymphoid Mild
proliferative
human CD34+stem chimerism providing Lungs, GN
cells to NOD SCID human neutrophil and kidneys
IL2-receptor KO monocyte targets for
mice followed by PR3-ANCA
passive transfer of
human PR3-ANCA
Modelling of anti-PR3 associated disease in animals is significantly less advanced and,
by virtue of the differences in PR3 structure and expression between mice and men, will
probably rely in the future on the use of humanized and transgenic in vivo systems. The
generation of an animal model incorporating both anti-PR3 associated vasculitis and
granuloma formation, as well as, relapsing disease, cardiac, gastrointestinal and neurologic
disease remains a major challenge for the next decade.
Complimentary Contributor Copy