Page 295 - The Vasculitides, Volume 1: General Considerations and Systemic Vasculitis
P. 295
Systemic Vasculitis and the Kidney 269
natural autoantibodies. Thus, while ANCA can be used to rule in disease in the appropriate
clinical setting, neither their presence nor absence alone can be used to determine if disease is
present. Additionally, some patients have persistently elevated autoantibody titers even in the
absence of any clinical evidence of disease, thereby limiting their utility in diagnosing disease
flares [64].
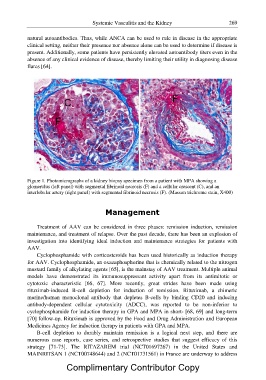
Figure 1. Photomicrographs of a kidney biopsy specimen from a patient with MPA showing a
glomerulus (left panel) with segmental fibrinoid necrosis (F) and a cellular crescent (C), and an
interlobular artery (right panel) with segmental fibrinoid necrosis (F). (Masson trichrome stain, X400)
Management
Treatment of AAV can be considered in three phases: remission induction, remission
maintenance, and treatment of relapse. Over the past decade, there has been an explosion of
investigation into identifying ideal induction and maintenance strategies for patients with
AAV.
Cyclophosphamide with corticosteroids has been used historically as induction therapy
for AAV. Cyclophosphamide, an oxazaphosphorine that is chemically related to the nitrogen
mustard family of alkylating agents [65], is the mainstay of AAV treatment. Multiple animal
models have demonstrated its immunosuppressant activity apart from its antimitotic or
cytotoxic characteristic [66, 67]. More recently, great strides have been made using
rituximab-induced B-cell depletion for induction of remission. Rituximab, a chimeric
murine/human monoclonal antibody that depletes B-cells by binding CD20 and inducing
antibody-dependent cellular cytotoxicity (ADCC), was reported to be non-inferior to
cyclophosphamide for induction therapy in GPA and MPA in short- [68, 69] and long-term
[70] follow-up. Rituximab is approved by the Food and Drug Administration and European
Medicines Agency for induction therapy in patients with GPA and MPA.
B-cell depletion to durably maintain remission is a logical next step, and there are
numerous case reports, case series, and retrospective studies that suggest efficacy of this
strategy [71-73]. The RITAZAREM trial (NCT01697267) in the United States and
MAINRITSAN 1 (NCT00748644) and 2 (NCT01731561) in France are underway to address
Complimentary Contributor Copy